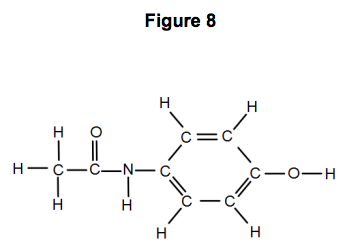
Acetyl salicylic acid (mol. wt. = 180) called aspirin is a pain killer with pKa = 6 . It two tablets each of 0.09 gm mass containing aspirin are dissolved in 100 mL solution. Its pH will be:
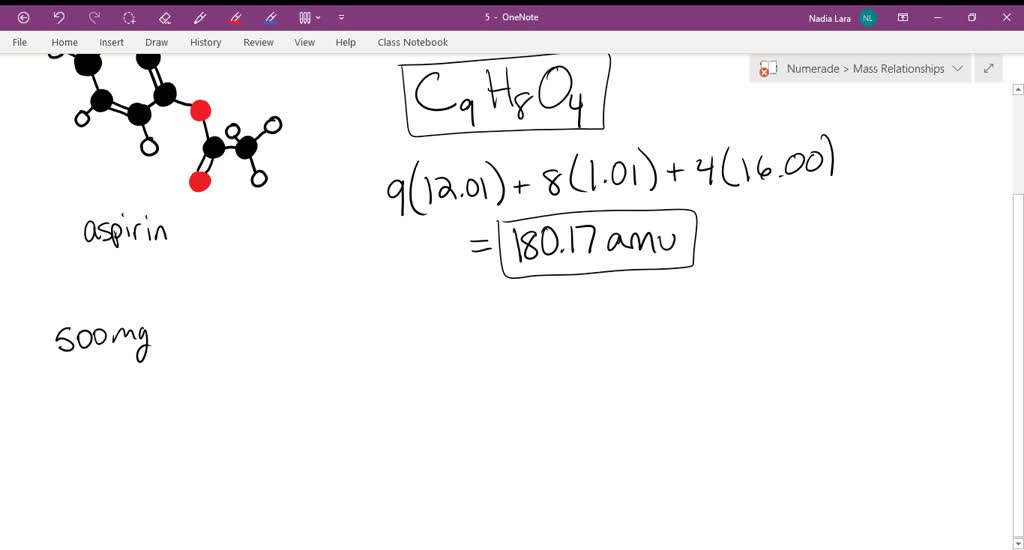
SOLVED:Aspirin can be represented by the adjacent ball-and-stick molecular model. Give the formula for aspirin, and calculate its molecular mass (red =O, gray =C, ivory =H ). How many moles of aspirin

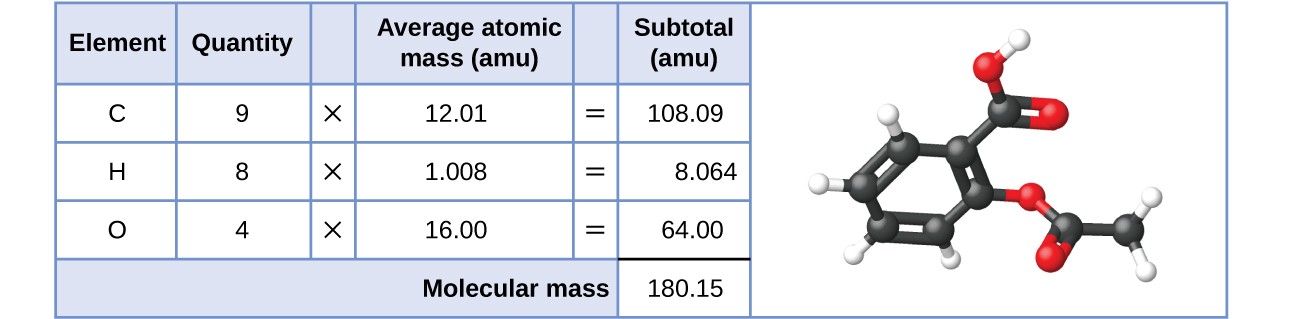
SOLVED:The molecular formula of acetylsalicylic acid (aspirin), one of the most commonly used pain relievers, is C9 H8 O4 a. Calculate the molar mass of aspirin. b. A typical aspirin tablet contains
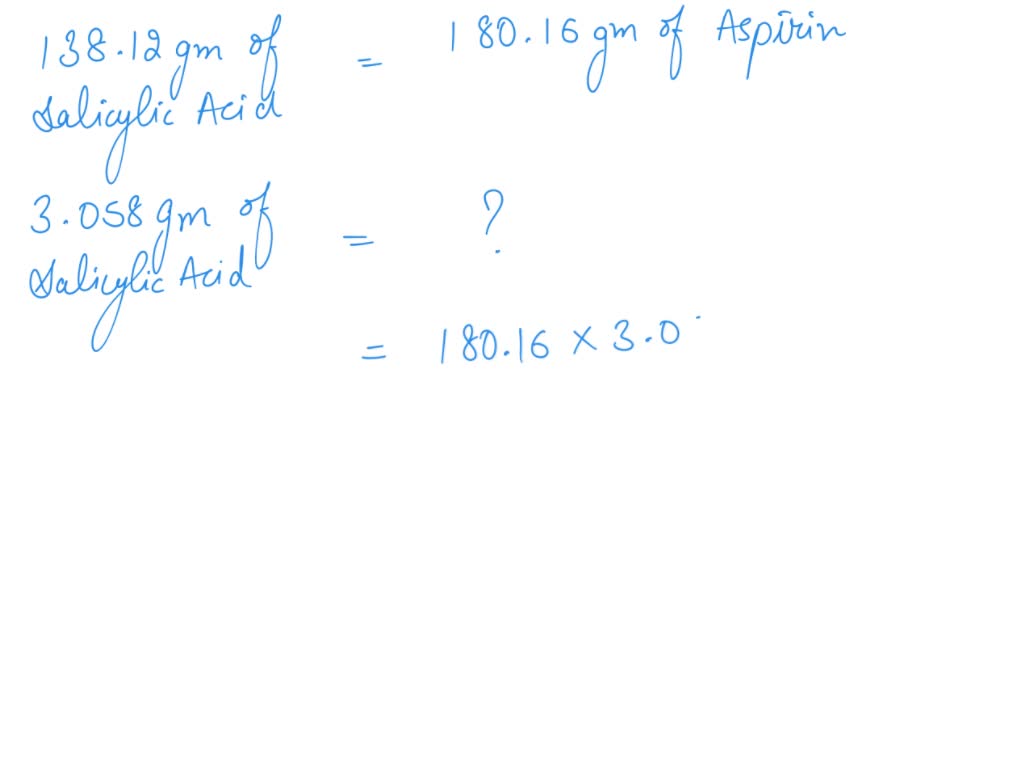
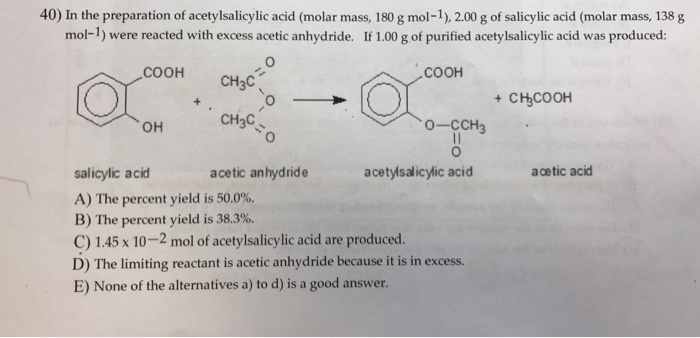
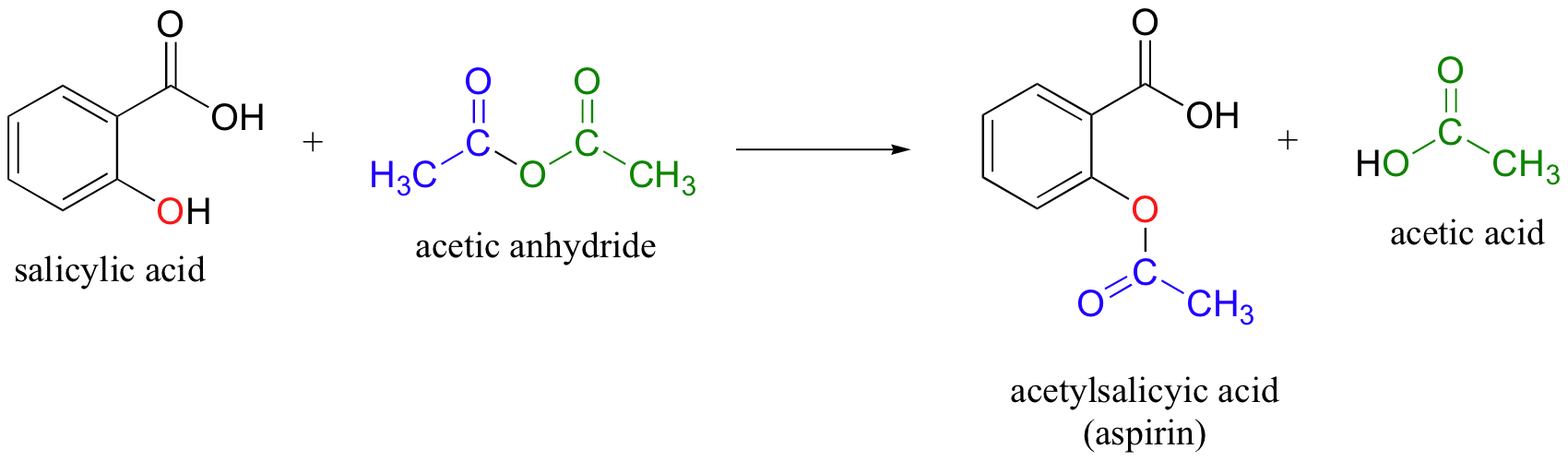
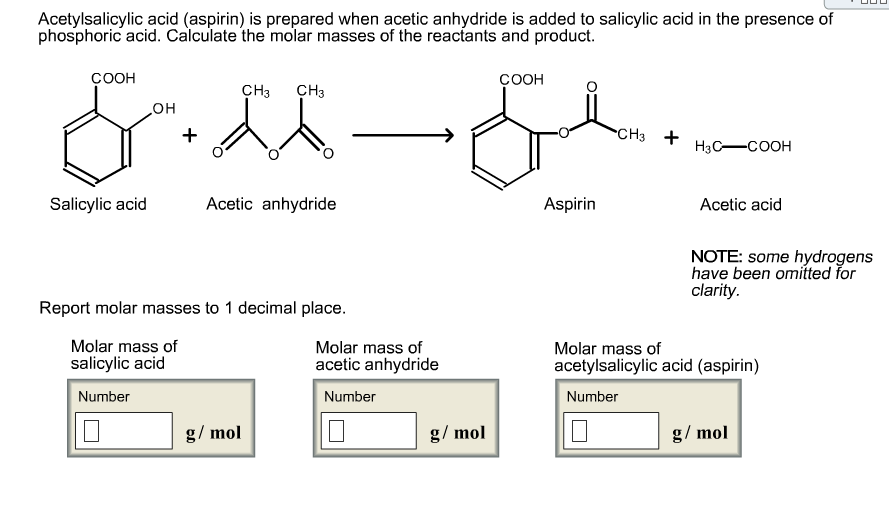
SOLVED: If one mole of salicylic acid (molar mass=138.12 g/mol) reacts with 1 mole of acetic anhydride (molar mass=102.09 g/mol) , it will yield 1 mole of aspirin (or acetylsalicylic acid; molar
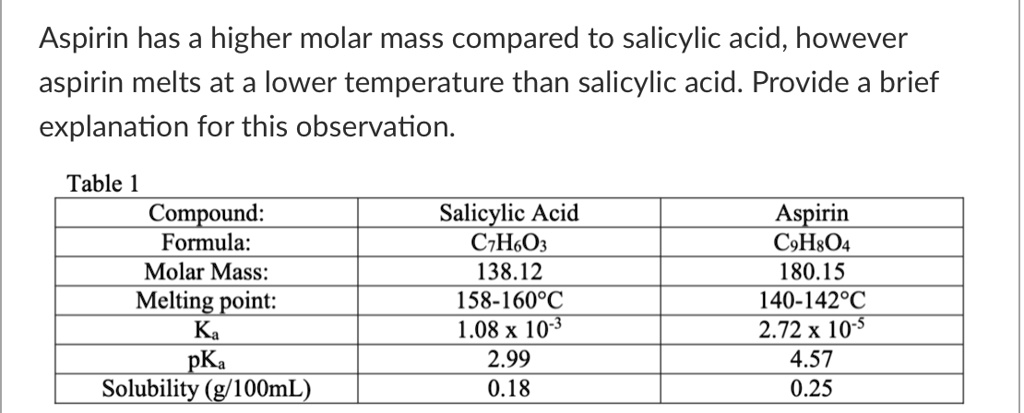
SOLVED: Aspirin has a higher molar mass compared to salicylic acid, however aspirin melts at a lower temperature than salicylic acid. Provide a brief explanation for this observation: Table 1 Compound: Formula: